FYARRO - Trademark Details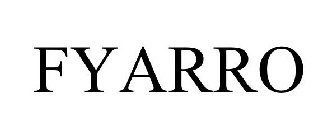
Status: 731 - Second Extension - Granted
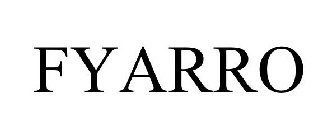
Serial Number
88599810
Word Mark
FYARRO
Status
731 - Second Extension - Granted
Status Date
2021-09-20
Filing Date
2019-08-30
Mark Drawing
4000 - Standard character mark
Typeset
Published for Opposition Date
2020-07-28
Attorney Name
Law Office Assigned Location Code
N30
Employee Name
BLACK, MILDRED ELIZABE
Statements
Goods and Services
Pharmaceutical preparations for the treatment and prevention of cancer, cardiovascular disease, epilepsy, mitochondrial disease, central nervous system disease, infectious disease, and acute respiratory disease; vaccine preparations; pharmaceutical preparations, namely, a drug formulation comprising nanoparticles of sirolimus and albumin for use in angiology, cardiology, endocrinology, gastroenterology, geriatrics, hematology, hepatology, nephrology, neurology, oncology, pulmonology, pneumology, respirology, rheumatology, anesthesiology, urology, dermatology, gynecology, ophthalmology, infectiology, and psychiatry; pharmaceutical preparations, namely, a drug formulation comprising nanoparticles of sirolimus and albumin for use in in pediatric, geriatric, emergency, family, and psychiatry; pharmaceutical preparations for the treatment of infectious, hereditary, and physiological diseases
Goods and Services
Medical and pharmaceutical research services; pharmaceutical research and development; providing medical and scientific research information in the field of pharmaceuticals and clinical trials; medical and scientific research, namely, conducting clinical trials for others; laboratory research services relating to pharmaceuticals; scientific and technological services, namely, research and design services in the field of process development, validation, qualification, and manufacturing of pharmaceuticals and biopharmaceuticals; scientific and technological services consultation services in the field of development, manufacturing and testing of pharmaceuticals and biopharmaceuticals; providing product research and development in the pharmaceutical field for purposes of process development, validation, qualification, and manufacturing of pharmaceuticals and biopharmaceuticals; scientific and technological consultation services in the field of pharmaceutical research and pharmacology; custom development of pharmaceutical, biopharmaceutical and chemical products; providing evaluation testing for pharmaceutical, biopharmaceutical and chemical products, and medical devices featuring testing for safety, toxicity, content, identity, purity, configuration and function, using chemical, in vitro or in vivo assays, analyses and models; pre-clinical and non-clinical evaluation testing of pharmaceutical, biopharmaceutical and chemical products performed primarily using animals and other biological models; pharmaceutical, biopharmaceutical, and chemical research and development for others; pharmaceutical research in the fields of angiology, cardiology, endocrinology, gastroenterology, geriatrics, hematology, hepatology, nephrology, neurology, oncology, pulmonology, pneumology, respirology, rheumatology, anesthesiology, urology, dermatology, gynecology, ophthalmology, infectiology, and psychiatry; pharmaceutical research for use in pediatric, geriatric, emergency, family, and preventative medicine; pharmaceutical research for the treatment of infectious, deficiency, hereditary, and physiological diseases
Classification Information
International Class
005 - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides. - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides.
US Class Codes
006, 018, 044, 046, 051, 052
Class Status Code
6 - Active
Class Status Date
2019-09-12
Primary Code
005
International Class
042 - Scientific and technological services and research and design relating thereto; industrial analysis and research services; design and development of computer hardware and software; legal services. - Scientific and technological services and research and design relating thereto; industrial analysis and research services; design and development of computer hardware and software; legal services.
US Class Codes
100, 101
Class Status Code
6 - Active
Class Status Date
2019-09-12
Primary Code
042
Current Trademark Owners
Party Name
Party Type
20 - Owner at Publication
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Trademark Owner History
Correspondences
Name
Aaron D. Hendelman
Address
Please log in with your Justia account to see this address.
Trademark Events
| Event Date | Event Description |
| 2019-09-03 | NEW APPLICATION ENTERED IN TRAM |
| 2019-09-12 | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM |
| 2019-12-04 | ASSIGNED TO EXAMINER |
| 2019-12-11 | NON-FINAL ACTION WRITTEN |
| 2019-12-11 | NON-FINAL ACTION E-MAILED |
| 2019-12-11 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2020-06-08 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2020-06-16 | ASSIGNED TO LIE |
| 2020-06-24 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2020-06-24 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2020-06-24 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| 2020-07-08 | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED |
| 2020-07-28 | PUBLISHED FOR OPPOSITION |
| 2020-07-28 | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED |
| 2020-09-22 | NOA E-MAILED - SOU REQUIRED FROM APPLICANT |
| 2021-02-23 | TEAS EXTENSION RECEIVED |
| 2021-02-23 | EXTENSION 1 FILED |
| 2021-02-23 | EXTENSION 1 GRANTED |
| 2021-02-25 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2021-09-20 | TEAS EXTENSION RECEIVED |
| 2021-09-20 | EXTENSION 2 FILED |
| 2021-09-20 | EXTENSION 2 GRANTED |
| 2021-09-22 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |