3D BIOLOGY - Trademark Details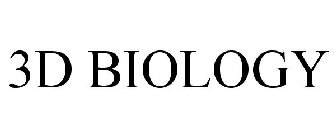
Status: 700 - Registered
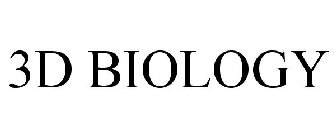
Serial Number
86725279
Registration Number
6130492
Word Mark
3D BIOLOGY
Status
700 - Registered
Status Date
2020-08-18
Filing Date
2015-08-14
Registration Number
6130492
Registration Date
2020-08-18
Mark Drawing
4000 - Standard character mark
Typeset
Published for Opposition Date
2017-04-25
Attorney Name
Law Office Assigned Location Code
M30
Employee Name
PERRY, KIMBERLY B
Statements
Disclaimer with Predetermined Text
"BIOLOGY"
Goods and Services
diagnostic reagents for scientific or research use in the field of cancer; diagnostic reagents for scientific research relating to molecular identification and digital quantification; diagnostic reagent kits comprising primarily diagnostic reagents for scientific or research use in the field of cancer; diagnostic test kits for scientific use comprising reagents, slides, solid matrix materials, microfluidic devices and support documentation, namely, instructional manuals, therefor; diagnostic test kits comprising reagents for scientific research relating to molecular identification and digital quantification; diagnostic reagents for in vitro use in biochemistry, clinical chemistry and microbiology; diagnostic reagents for clinical or medical laboratory use, namely, reagents for proteomics, RNA transcription and microRNA expression profiling, small-molecule analysis, single nucleotide polymorphisms analysis, DNA sequencing, detection of polymorphisms in human fluids, detection of copy number variations, and clinical, oncology, and hematology diagnostics; multiparameter quantitative detection and analysis products and technology for research and diagnostics for a variety of uses in the health care and other life science sectors, namely, reagents for scientific or medical research use, diagnostic preparations and reagents for scientific or research uses, namely, RNA transcription profiling, proteomics, clinical diagnostics, environmental monitoring, biosurveillance, agricultural inspection, forensic testing of samples containing microorganisms or human fluids, small-molecule analysis, single nucleotide polymorphisms analysis, and DNA sequencing; test kits, consisting of chemical preparations for scientific purposes, in particular for the detection, diagnosis, progress monitoring and progress prediction of diseases, including inflammations, infections, disorders of the central nervous systems, cardiovascular, neurological, endocrine, autoimmune and genetic disorders and cancers; assays, reagents, enzymes, polymerases, buffers and nucleotides, and kits comprised thereof, all for scientific or research use; multiparameter quantitative detection and analysis products and technology for research and diagnostics for a variety of uses in life science sectors, namely, diagnostic reagents for scientific in vitro use in biochemistry, clinical chemistry and microbiology, RNA and microRNA expression transcription profiling, proteomics, clinical diagnostics, environmental monitoring, agricultural inspection, small-molecule analysis, single nucleotide polymorphisms analysis, and DNA sequencing
Goods and Services
diagnostic reagents for medical use in the field of cancer; diagnostic reagent kits comprising primarily of diagnostic reagents for medical use in the field of cancer; chemical reagents for medical use; diagnostic test kits for medical use comprising primarily reagents, slides, solid matrix materials, microfluidic devices sold as a unit with instructional manuals therefor; medical diagnostic test kits for detecting pathogens, comprising primarily reagents, slides, solid matrix materials, microfluidic devices sold as a unit and instructional manuals therefor; medical diagnostic kits comprising medical diagnostic reagents for use in detecting the presence of analytes in samples and identifying sample type, and equipment, namely, medical diagnostic immuno-assays for screening samples detecting the presence of analytes in samples and identifying sample type, sold as a unit with instructional manuals; replacement parts for medical diagnostic kits for use in detecting the presence of analytes in samples and identifying sample type in the nature of equipment, namely, medical diagnostic immune-assays for screening samples detecting the presence of analytes in samples and identifying sample type; medical diagnostic reagents for RNA transcription and microRNA expression profiling, proteomics, small molecule analysis, single nucleotide polymorphisms analysis, DNA sequencing, detection of polymorphisms in human fluids, detection of copy number variations, and clinical, oncology, and hematology diagnostics; multiparameter quantitative detection and analysis products and technology for research and diagnostics for a variety of uses in the health care, namely, medical diagnostic reagents and diagnostic reagents for clinical or medical laboratory use, diagnostic reagents for medical in vitro use in biochemistry, clinical chemistry and microbiology, RNA and microRNA expression transcription profiling, proteomics, clinical diagnostics, environmental monitoring, agricultural inspection, small-molecule analysis, single nucleotide polymorphisms analysis, and DNA sequencing; tests designed to isolate and extract cells for genetic analysis, namely, reagents for medical diagnostic use; medical diagnostic reagents and assays for testing of body fluids; kits consisting primarily of medical diagnostic reagents and assays for testing of body fluids; reagents in the form of cell markers and molecule markers for medical purposes
Goods and Services
research and product development services for others in the field of biotechnology; providing laboratory research services in the field of gene expression; genetic data mining; providing molecule identification methods for acquiring, analyzing and managing biological and genetic information in the field of genetics; research, design, and development services for others in the fields of diagnostic reagents
Pseudo Mark
THREE D BIOLOGY
Classification Information
International Class
042 - Scientific and technological services and research and design relating thereto; industrial analysis and research services; design and development of computer hardware and software; legal services. - Scientific and technological services and research and design relating thereto; industrial analysis and research services; design and development of computer hardware and software; legal services.
US Class Codes
100, 101
Class Status Code
6 - Active
Class Status Date
2015-08-19
Primary Code
042
First Use Anywhere Date
2017-04-17
First Use In Commerce Date
2017-04-17
Current Trademark Owners
Party Name
Party Type
30 - Original Registrant
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Trademark Owner History
Party Name
Party Type
30 - Original Registrant
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
20 - Owner at Publication
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
10 - Original Applicant
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Correspondences
Name
Alyssa M. Worsham
Address
Please log in with your Justia account to see this address.
Trademark Events
| Event Date | Event Description |
| 2015-08-18 | NEW APPLICATION ENTERED IN TRAM |
| 2015-08-19 | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM |
| 2015-08-20 | NOTICE OF PSEUDO MARK E-MAILED |
| 2015-11-30 | ASSIGNED TO EXAMINER |
| 2015-12-02 | NON-FINAL ACTION WRITTEN |
| 2015-12-02 | NON-FINAL ACTION E-MAILED |
| 2015-12-02 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2016-06-21 | TEAS PETITION TO REVIVE RECEIVED |
| 2016-06-21 | PETITION TO REVIVE-GRANTED |
| 2016-06-21 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2016-06-22 | NOTICE OF REVIVAL - E-MAILED |
| 2016-06-28 | ASSIGNED TO LIE |
| 2016-06-29 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2016-06-29 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2016-07-11 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| 2016-07-18 | LAW OFFICE PUBLICATION REVIEW COMPLETED |
| 2016-08-02 | WITHDRAWN FROM PUB - OG REVIEW QUERY |
| 2016-08-24 | PREVIOUS ALLOWANCE COUNT WITHDRAWN |
| 2016-08-31 | NON-FINAL ACTION WRITTEN |
| 2016-08-31 | NON-FINAL ACTION E-MAILED |
| 2016-08-31 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2017-02-28 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2017-02-28 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2017-03-03 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2017-03-13 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| 2017-03-21 | LAW OFFICE PUBLICATION REVIEW COMPLETED |
| 2017-04-05 | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED |
| 2017-04-25 | PUBLISHED FOR OPPOSITION |
| 2017-04-25 | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED |
| 2017-06-20 | NOA E-MAILED - SOU REQUIRED FROM APPLICANT |
| 2017-12-18 | TEAS EXTENSION RECEIVED |
| 2017-12-18 | EXTENSION 1 FILED |
| 2017-12-18 | EXTENSION 1 GRANTED |
| 2017-12-20 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2018-06-15 | TEAS EXTENSION RECEIVED |
| 2018-06-15 | EXTENSION 2 FILED |
| 2018-06-15 | EXTENSION 2 GRANTED |
| 2018-06-19 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2018-12-13 | TEAS EXTENSION RECEIVED |
| 2018-12-13 | EXTENSION 3 FILED |
| 2018-12-13 | EXTENSION 3 GRANTED |
| 2018-12-15 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2019-06-12 | TEAS EXTENSION RECEIVED |
| 2019-06-12 | EXTENSION 4 FILED |
| 2019-06-12 | EXTENSION 4 GRANTED |
| 2019-06-14 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2019-11-14 | TEAS REVOKE/APP/CHANGE ADDR OF ATTY/DOM REP RECEIVED |
| 2019-11-14 | ATTORNEY/DOM.REP.REVOKED AND/OR APPOINTED |
| 2019-11-25 | TEAS EXTENSION RECEIVED |
| 2019-12-02 | CASE ASSIGNED TO INTENT TO USE PARALEGAL |
| 2019-11-25 | EXTENSION 5 FILED |
| 2019-12-02 | EXTENSION 5 GRANTED |
| 2019-12-03 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2020-06-17 | TEAS STATEMENT OF USE RECEIVED |
| 2020-06-17 | USE AMENDMENT FILED |
| 2020-06-22 | STATEMENT OF USE PROCESSING COMPLETE |
| 2020-07-16 | ALLOWED PRINCIPAL REGISTER - SOU ACCEPTED |
| 2020-07-17 | NOTICE OF ACCEPTANCE OF STATEMENT OF USE E-MAILED |
| 2020-08-18 | REGISTERED-PRINCIPAL REGISTER |