IVXX ELEVATE - Trademark Details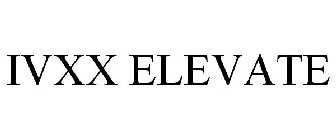
Status: 645 - Final Refusal - Mailed
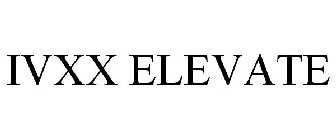
Serial Number
86400760
Word Mark
IVXX ELEVATE
Status
645 - Final Refusal - Mailed
Status Date
2022-02-03
Filing Date
2014-09-19
Mark Drawing
4000 - Standard character mark
Typeset
Attorney Name
Law Office Assigned Location Code
L80
Employee Name
BEN, LINDSEY HEATHER
Statements
Goods and Services
Goods permitted by the Farm Bill and not subject to the FDCA: Medicated beverages, Medicated sodas, Medicated candy, Medicated chewing gum, Medicated confectionery, Medicinal drinks, Medicinal herb extracts, Medicinal herbal preparations, Medicinal herbs in dried or preserved form, Medicinal preparations for the mouth to be applied in the form of drops, capsules, tablets and compressed tablets, Sweets for medicinal purposes, Balms for medical purposes, Herbal topical creams, gels, salves, sprays, powder, balms, liniment and ointments for the relief of aches and pain, all of the foregoing containing cannabis, hemp, industrial hemp, CBD, or cannabidiol oil with a delta-9 THC concentration of not more than 0.3% on a dry weight basis; Medicated beverages, Medicated sodas, Medicated candy, Medicated chewing gum, Medicated confectionery, Medicinal drinks, Medicinal herb extracts, Medicinal herbal preparations, Medicinal herbs in dried or preserved form, Medicinal preparations for the mouth to be applied in the form of drops, capsules, tablets and compressed tablets, Sweets for medicinal purposes, Balms for medical purposes, Herbal topical creams, gels, salves, sprays, powder, balms, liniment and ointments for the relief of aches and pain, all of the aforementioned goods with a delta-9 tetrahydrocannabinol THC concentration of not more than 0.3 percent on a dry weight basis and not containing CBD; Goods that do not contain any reference to cannabis, hemp, industrial hemp, CBD, or cannabidiol oil: Medicated beverages, Medicated sodas, Medicated candy, Medicated chewing gum, Medicated confectionery, Medicinal drinks, Medicinal herb extracts, Medicinal herbal preparations, Medicinal herbs in dried or preserved form, Medicinal preparations for the mouth to be applied in the form of drops, capsules, tablets and compressed tablets, Sweets for medicinal purposes, Balms for medical purposes, Herbal topical creams, gels, salves, sprays, powder, balms, liniment and ointments for the relief of aches and pain, none of the foregoing containing cannabis, hemp, industrial hemp, CBD, or cannabidiol oil; Goods not subject to CSA or FDCA: Medicated beverages, Medicated sodas, Medicated candy, Medicated chewing gum, Medicated confectionery, Medicinal drinks, Medicinal herb extracts, Medicinal herbal preparations, Medicinal herbs in dried or preserved form, Medicinal preparations for the mouth to be applied in the form of drops, capsules, tablets and compressed tablets, Sweets for medicinal purposes, Balms for medical purposes, Herbal topical creams, gels, salves, sprays, powder, balms, liniment and ointments for the relief of aches and pain, all of the foregoing being comprised in significant part of sterilized hemp seeds, mature stalk and containing no other cannabis ingredients; Medicated beverages, Medicated sodas, Medicated candy, Medicated chewing gum, Medicated confectionery, Medicinal drinks, Medicinal herb extracts, Medicinal herbal preparations, Medicinal herbs in dried or preserved form, Medicinal preparations for the mouth to be applied in the form of drops, capsules, tablets and compressed tablets, Sweets for medicinal purposes, Balms for medical purposes, Herbal topical creams, gels, salves, sprays, powder, balms, liniment and ointments for the relief of aches and pain, all of the foregoing containing hulled hemp seeds, hemp seed protein and hemp seed oil; Medicated beverages, Medicated sodas, Medicated candy, Medicated chewing gum, Medicated confectionery, Medicinal drinks, Medicinal herb extracts, Medicinal herbal preparations, Medicinal herbs in dried or preserved form, Medicinal preparations for the mouth to be applied in the form of drops, capsules, tablets and compressed tablets, Sweets for medicinal purposes, Balms for medical purposes, Herbal topical creams, gels, salves, sprays, powder, balms, liniment and ointments for the relief of aches and pain, all of the foregoing containing CBD that contains no more than 0.3 % THC on a dry weight basis; Medicated beverages, Medicated sodas, Medicated candy, Medicated chewing gum, Medicated confectionery, Medicinal drinks, Medicinal herb extracts, Medicinal herbal preparations, Medicinal herbs in dried or preserved form, Medicinal preparations for the mouth to be applied in the form of drops, capsules, tablets and compressed tablets, Sweets for medicinal purposes, Balms for medical purposes, Herbal topical creams, gels, salves, sprays, powder, balms, liniment and ointments for the relief of aches and pain, all of the foregoing containing CBD derived from industrial hemp extracted solely from the mature stalks and sterilized seeds; Medicated beverages containing cannabidiol oil and CBD derived from industrial hemp; Medicated sodas containing cannabidiol oil and CBD derived from industrial hemp; Medicated candy containing cannabidiol oil and CBD derived from industrial hemp; Medicated chewing gum containing cannabidiol oil and CBD derived from industrial hemp; Medicated confectionery containing cannabidiol oil and CBD derived from industrial hemp; Medicinal drinks containing cannabidiol oil and CBD derived from industrial hemp; Medicinal herb extracts containing cannabidiol oil and CBD derived from industrial hemp; Medicinal herbal preparations containing cannabidiol oil and CBD derived from industrial hemp; Medicinal herbs in dried or preserved form containing cannabidiol oil and CBD derived from industrial hemp; Medicinal preparations for the mouth to be applied in the form of drops, capsules, tablets and compressed tablets containing cannabidiol oil and CBD derived from industrial hemp; Sweets for medicinal purposes containing cannabidiol oil and CBD derived from industrial hemp; Balms for medical purposes containing cannabidiol oil and CBD derived from industrial hemp; Herbal topical creams, gels, salves, sprays, powder, balms, liniment and ointments for the relief of aches and pain containing cannabidiol oil and CBD derived from industrial hemp; Medicated beverages containing industrial hemp; Medicated sodas containing industrial hemp; Medicated candy containing industrial hemp; Medicated chewing gum containing industrial hemp; Medicated confectionery containing industrial hemp; Medicinal drinks containing industrial hemp; Medicinal herb extracts containing industrial hemp; Medicinal herbal preparations containing industrial hemp; Medicinal herbs in dried or preserved form containing industrial hemp; Medicinal preparations for the mouth to be applied in the form of drops, capsules, tablets and compressed tablets containing industrial hemp; Sweets for medicinal purposes containing industrial hemp; Balms for medical purposes containing industrial hemp; Herbal topical creams, gels, salves, sprays, powder, balms, liniment and ointments for the relief of aches and pain containing industrial hemp; Medicated beverages containing hemp; Medicated sodas containing hemp; Medicated candy containing hemp; Medicated chewing gum containing hemp; Medicated confectionery containing hemp; Medicinal drinks containing hemp; Medicinal herb extracts containing hemp; Medicinal herbal preparations containing hemp; Medicinal herbs in dried or preserved form containing hemp; Medicinal preparations for the mouth to be applied in the form of drops, capsules, tablets and compressed tablets containing hemp; Sweets for medicinal purposes containing hemp; Balms for medical purposes containing hemp; Herbal topical creams, gels, salves, sprays, powder, balms, liniment and ointments for the relief of aches and pain containing hemp; Goods potentially subject to CSA and the FDCA: Medicated beverages containing cannabis; Medicated sodas containing cannabis; Medicated candy containing cannabis; Medicated chewing gum containing cannabis; Medicated confectionery containing cannabis; Medicinal drinks containing cannabis; Medicinal herb extracts containing cannabis; Medicinal herbal preparations containing cannabis; Medicinal herbs in dried or preserved form containing cannabis; Medicinal preparations for the mouth to be applied in the form of drops, capsules, tablets and compressed tablets containing cannabis; Sweets for medicinal purposes containing cannabis; Balms for medical purposes containing cannabis; Herbal topical creams, gels, salves, sprays, powder, balms, liniment and ointments for the relief of aches and pain containing cannabis; Medicated beverages containing CBD; Medicated sodas containing CBD; Medicated candy containing CBD; Medicated chewing gum containing CBD; Medicated confectionery containing CBD; Medicinal drinks containing CBD; Medicinal herb extracts containing CBD; Medicinal herbal preparations containing CBD; Medicinal herbs in dried or preserved form containing CBD; Medicinal preparations for the mouth to be applied in the form of drops, capsules, tablets and compressed tablets containing CBD; Sweets for medicinal purposes containing CBD; Balms for medical purposes containing CBD; Herbal topical creams, gels, salves, sprays, powder, balms, liniment and ointments for the relief of aches and pain containing CBD
Goods and Services
Goods permitted by the Farm Bill and not subject to the FDCA: Brownies, Chocolate and chocolates, Chocolate confections, cookies, and candy, all of the foregoing containing cannabis, hemp, industrial hemp, CBD, or cannabidiol oil with a delta-9 THC concentration of not more than 0.3% on a dry weight basis; Brownies, Chocolate and chocolates, Chocolate confections, cookies, and candy all of the aforementioned goods with a delta-9 tetrahydrocannabinol THC concentration of not more than 0.3 percent on a dry weight basis and not containing CBD; Goods that do not contain any reference to cannabis, hemp, industrial hemp, CBD, or cannabidiol oil: Brownies, Chocolate and chocolates, Chocolate confections, cookies, candy, none of the foregoing containing cannabis, hemp, industrial hemp, CBD, or cannabidiol oil; Goods not subject to CSA or FDCA: Brownies, Chocolate and chocolates, Chocolate confections, cookies, candy, all of the foregoing being comprised in significant part of sterilized hemp seeds, mature stalk and containing no other cannabis ingredients; Brownies, Chocolate and chocolates, Chocolate confections, cookies, candy, all of the foregoing containing hulled hemp seeds, hemp seed protein and hemp seed oil; Brownies, Chocolate and chocolates, Chocolate confections, cookies, candy, all of the foregoing containing CBD that contains no more than 0.3 % THC on a dry weight basis; Brownies, Chocolate and chocolates, Chocolate confections, cookies, candy, all of the foregoing containing CBD derived from industrial hemp extracted solely from the mature stalks and sterilized seeds; Brownies, Chocolate and chocolates, Chocolate confections, cookies, candy, all of the foregoing containing cannabidiol oil and CBD derived from industrial hemp; Brownies, Chocolate and chocolates, Chocolate confections, cookies, candy containing industrial hemp; Brownies, Chocolate and chocolates, Chocolate confections, cookies, candy containing hemp; Goods potentially subject to CSA and the FDCA: Brownies, Chocolate and chocolates, Chocolate confections, cookies, candy containing cannabis; Brownies, Chocolate and chocolates, Chocolate confections, cookies, candy containing CBD
Classification Information
International Class
005 - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides. - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides.
US Class Codes
006, 018, 044, 046, 051, 052
Class Status Code
6 - Active
Class Status Date
2014-09-29
Primary Code
005
International Class
030 - Coffee, tea, cocoa, sugar, rice, tapioca, sago, artificial coffee; flour and preparations made from cereals, bread, pastry and confectionery, ices; honey, treacle; yeast, baking-powder; salt, mustard; vinegar, sauces (condiments); spices; ice. - Coffee, tea, cocoa, sugar, rice, tapioca, sago, artificial coffee; flour and preparations made from cereals, bread, pastry and confectionery, ices; honey, treacle; yeast, baking-powder; salt, mustard; vinegar, sauces (condiments); spices; ice.
US Class Codes
046
Class Status Code
6 - Active
Class Status Date
2016-02-23
Primary Code
030
Current Trademark Owners
Party Name
Party Type
10 - Original Applicant
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Correspondences
Name
Jonathan A. Hyman
Address
Please log in with your Justia account to see this address.
Prior Registrations
| Relationship Type | Reel Number |
| Prior Registration | 4400287 |
| Prior Registration | 4893219 |
| Prior Registration | 6153426 |
Trademark Events
| Event Date | Event Description |
| 2014-09-23 | NEW APPLICATION ENTERED IN TRAM |
| 2014-09-29 | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM |
| 2014-12-30 | ASSIGNED TO EXAMINER |
| 2015-01-07 | ASSIGNED TO EXAMINER |
| 2015-01-08 | NON-FINAL ACTION WRITTEN |
| 2015-01-08 | NON-FINAL ACTION E-MAILED |
| 2015-01-08 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2015-07-08 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2015-07-08 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2015-07-09 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2015-08-04 | NON-FINAL ACTION WRITTEN |
| 2015-08-04 | NON-FINAL ACTION E-MAILED |
| 2015-08-04 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2016-02-04 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2016-02-04 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2016-02-05 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2016-02-23 | NON-FINAL ACTION WRITTEN |
| 2016-02-23 | NON-FINAL ACTION E-MAILED |
| 2016-02-23 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2016-08-23 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2016-08-23 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2016-08-24 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2016-09-20 | NON-FINAL ACTION WRITTEN |
| 2016-09-20 | NON-FINAL ACTION E-MAILED |
| 2016-09-20 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2017-03-20 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2017-03-20 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2017-03-21 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2017-05-17 | ASSIGNED TO EXAMINER |
| 2017-06-15 | TEAS CHANGE OF OWNER ADDRESS RECEIVED |
| 2017-06-15 | APPLICANT/CORRESPONDENCE CHANGES (NON-RESPONSIVE) ENTERED |
| 2017-06-22 | NON-FINAL ACTION WRITTEN |
| 2017-06-22 | NON-FINAL ACTION E-MAILED |
| 2017-06-22 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2017-12-22 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2017-12-22 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2017-12-23 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2018-01-23 | ASSIGNED TO EXAMINER |
| 2018-01-29 | NON-FINAL ACTION WRITTEN |
| 2018-01-29 | NON-FINAL ACTION E-MAILED |
| 2018-01-29 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2018-07-30 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2018-07-30 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2018-07-31 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2018-08-23 | NON-FINAL ACTION WRITTEN |
| 2018-08-23 | NON-FINAL ACTION E-MAILED |
| 2018-08-23 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2019-02-25 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2019-02-25 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2019-02-26 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2019-05-15 | NON-FINAL ACTION WRITTEN |
| 2019-05-15 | NON-FINAL ACTION E-MAILED |
| 2019-05-15 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2019-11-14 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2019-11-14 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2019-11-15 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2020-01-14 | NON-FINAL ACTION WRITTEN |
| 2020-01-14 | NON-FINAL ACTION E-MAILED |
| 2020-01-14 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2020-07-14 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2020-07-14 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2020-07-15 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2020-08-06 | NON-FINAL ACTION WRITTEN |
| 2020-08-06 | NON-FINAL ACTION E-MAILED |
| 2020-08-06 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2021-02-05 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2021-02-05 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2021-02-05 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2021-04-13 | NON-FINAL ACTION WRITTEN |
| 2021-04-13 | NON-FINAL ACTION E-MAILED |
| 2021-04-13 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2021-10-13 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2021-10-13 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2021-10-14 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2022-02-03 | FINAL REFUSAL WRITTEN |
| 2022-02-03 | FINAL REFUSAL E-MAILED |
| 2022-02-03 | NOTIFICATION OF FINAL REFUSAL EMAILED |