MOLEQ - Trademark Details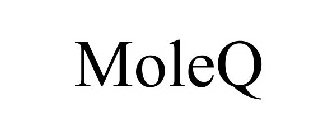
Status: 701 - Section 8-Accepted
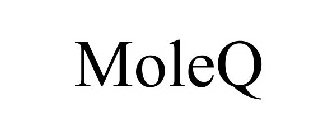
Serial Number
86058414
Registration Number
4536664
Word Mark
MOLEQ
Status
701 - Section 8-Accepted
Status Date
2020-09-09
Filing Date
2013-09-07
Registration Number
4536664
Registration Date
2014-05-27
Mark Drawing
4000 - Standard character mark
Typeset
Published for Opposition Date
2014-03-11
Attorney Name
Law Office Assigned Location Code
L20
Employee Name
COLEMAN, CIMMERIAN
Statements
Indication of Colors claimed
Color is not claimed as a feature of the mark.
Goods and Services
[ Albumin dietary supplements; Alginate dietary supplements; Alkalinity buffer supplements for live coral for use in aquariums; Amino acids for nutritional purposes; Animal feed additive for use as a nutritional supplement for medical purposes; Animal feed supplements; Antioxidant enriched coffee; Bee pollen for use as a dietary food supplement; Beverages containing chlorophyll for use as a nutritional supplement; Calcium montmorillonite clay for therapeutic purposes used to enhance the production of enzymes in living beings or as a mineral supplement; Calcium supplements; Calcium-based nutrient supplements for live coral for use in aquariums; Casein dietary supplements; ] Dietary and nutritional supplements; [ Dietary and nutritional supplements for endurance sports; Dietary and nutritional supplements used for weight loss; Dietary beverage supplements for human consumption in liquid and dry mix form for therapeutic purposes; ] Dietary food supplements; [ Dietary pet supplements in the form of pet treats; Dietary supplement drink mixes; Dietary supplement for eliminating toxins from the intestinal tract; Dietary supplemental drinks; Dietary supplemental drinks in the nature of vitamin and mineral beverages; ] Dietary supplements; [ Dietary supplements for animals; Dietary supplements for controlling cholesterol; ] Dietary supplements for human consumption; [ Dietary supplements for pets; Dietary supplements for pets in the nature of a powdered drink mix; Dietary supplements for treatment of claustrophobia; Dietary supplements for urinary health; Dietary supplements in the nature of weight loss powders; Drug delivery agents consisting of compounds that facilitate delivery of a wide range of pharmaceuticals; Drug delivery agents in the form of capsules that provide controlled release of the active ingredients for a wide variety of pharmaceuticals; Drug delivery agents in the form of powders that provide controlled release of the active ingredients for a wide variety of pharmaceuticals; Drug delivery agents in the form of tablets that provide controlled release of the active ingredients for a wide variety of pharmaceuticals; Drug testing kits comprised of medical diagnostic reagents and assays for testing body fluids;] Enzyme dietary supplements; Enzyme food supplements; [ Flaxseed dietary supplements; Flaxseed oil dietary supplements; ] Food supplements; Food supplements, namely, anti-oxidants; [ Glucose dietary supplements; Ground flaxseed fiber for use as a dietary supplement; Health food supplements; Herbal supplements; Herbal supplements for sleeping problems; Homeopathic supplements; Human allograft bone and tissue; Human allograft tissue; Human growth hormone; Intravenous fluids used for rehydration, nut; rition and the delivery of pharmaceutical preparations; Lecithin for use as a dietary supplement; Linseed dietary supplements; Linseed oil dietary supplements; Liquid herbal supplements; ] Liquid nutritional supplement; [ Liquid protein supplements; Liquid vitamin supplements; Medical adhesive tape; Medical adhesives for binding internal tissue; Medical adhesives for binding wounds; Medical and surgical dressings; Medical and surgical plasters; Medical cleansers for skin and wounds; Medical diagnostic reagents; Medical diagnostic reagents and assays for testing body fluids for microorganisms; Medical diagnostic reagents and assays for testing of body fluids; Medical diagnostic reagents for the analysis of body fluids; Medical dressings; Medical lubricant, namely, vaginal lubricants; Medical plasters; Medical preparations for slimming purposes; Medical preparations, namely, foot, hand and skin creams for diabetics; Medicated supplements for foodstuffs for animals; Medicated supplements for foodstuffs for babies; Medicinal alcohol; Medicinal creams for skin care; Medicinal drinks; Medicinal hair growth preparations; Medicinal herb extracts; Medicinal herbal extracts for medical purposes; Medicinal herbal preparations; Medicinal herbs; Medicinal herbs in dried or preserved form; Medicinal oils; Medicinal preparations for stimulating hair growth; Medicinal preparations for the mouth and as sprays; Medicinal preparations for the mouth to be applied in the form of drops, capsules, tablets and compressed tablets; Medicinal preparations for the treatment of infectious diseases and for use in oncology; Medicinal radix glycyrrhizae; Medicinal roots; Medicinal tea; Medicines for dental purposes; Medicines for the treatment of gastrointestinal diseases; Mineral food supplements; Mineral nutritional supplements; Mineral supplements; Natural dietary supplements for treatment of claustrophobia; Natural herbal supplements; Natural supplements for treating candida; Natural supplements for treating depression and anxiety; Natural supplements for treating erectile dysfunction; Non-medicated additives for animal feed for use as nutritional supplements; Nopal cactus juice for use as a nutritional supplement; Nutraceuticals for use as a dietary supplement; Nutritional and dietary supplements formed and packaged as bars; Nutritional drinks for animals; Nutritional meal replacement bars adapted for medical use for individuals undergoing medical treatments; Nutritional supplement energy bars; Nutritional supplement for eliminating toxins from the body; Nutritional supplement for eliminating toxins from the intestinal tract; Nutritional supplement in the nature of a nutrient-dense, protein-based drink mix; Nutritional supplement meal replacement bars for boosting energy; Nutritional supplement shakes; ] Nutritional supplements; [ Nutritional supplements in capsule form for dogs; Nutritional supplements in lotion form sold as a component of nutritional skin care products; Nutritional supplements in the nature of nutritionally fortified soft chews; Nutritional supplements, namely, carbohydrates in powdered form; Nutritional supplements, namely, probiotic compositions; Nutritive substances for micro-organisms for medical use; Nutritive substances for microorganism cultures; Nutritive substances for microorganisms for medical purposes; Pharmaceutical preparation for skin care; Pharmaceutical preparation for the treatment of gout; Pharmaceutical preparations acting on the central nervous system; Pharmaceutical preparations and substances for the treatment of damaged skin and tissue; Pharmaceutical preparations and substances for the treatment of gastro-intestinal diseases; Pharmaceutical preparations and substances for the treatment of infectious diseases, blood disorders, pain, inflammation, sepsis, alopecia, obesity and cognitive disorders; Pharmaceutical preparations and substances for the treatment of psychiatric diseases and disorders; Pharmaceutical preparations and substances for the treatment of viral, metabolic, endocrine, musculoskeletal, cardiovascular, cardiopulmonary, genitourinary, sexual dysfunction, oncological, hepatological, ophthalmic, respiratory, neurological, gastrointestinal, hormonal, dermatological, psychiatric and immune system related diseases and disorders; Pharmaceutical preparations for animal skincare; Pharmaceutical preparations for inhalation for the treatment of pulmonary hypertension; Pharmaceutical preparations for ocular or intraocular surgery; Pharmaceutical preparations for peripheral nervous system; Pharmaceutical preparations for reducing sexual activity; Pharmaceutical preparations for skin care; Pharmaceutical preparations for the prevention and treatment of disorders of the nervous system, the immune system, the cardio-vascular system, the metabolic system, the respiratory system, the musculo-skeletal system, the genitourinary system; for the treatment of inflammatory disorders; for use in dermatology, oncology, hematology and in tissue and organ transplantation, in ophthalmology and for gastroenterological disorders; Pharmaceutical preparations for the prevention and treatment of ocular disorders or diseases, bacteria-based diseases or disorders, autoimmune diseases or disorders, kidney diseases or disorders, and diabetes; Pharmaceutical preparations for the prevention and treatment of ocular disorders or diseases, for the treatment of bacteria-based diseases, and for the treatment of diabetes, and anti - infective preparations, antiviral preparations, antibiotics, antifungal preparations and vaccines; Pharmaceutical preparations for the treatment of eye diseases and conditions; Pharmaceutical preparations for the treatment of heart rhythm disorders; Pharmaceutical preparations for the treatment of hormonal disorders and the prevention of osteoporosis; Pharmaceutical preparations for the treatment of immune system related diseases and disorders; Pharmaceutical preparations for the treatment of infectious diseases; Pharmaceutical preparations for the treatment of kidney diseases; Pharmaceutical preparations for the treatment of worms in pets; Pharmaceutical preparations for treating allergic rhinitis and asthma; Pharmaceutical preparations for treating allergies; Pharmaceutical preparations for treating and preventing tendon and muscle injuries and disorders, sports related injuries, and for knee cartilage regeneration; Pharmaceutical preparations for treating dandruff; Pharmaceutical preparations for treating diabetes; Pharmaceutical preparations for treating hypertension; Pharmaceutical preparations for treating skin disorders; Pharmaceutical preparations for use in chemotherapy; Pharmaceutical preparations for use in dermatology; Pharmaceutical preparations for use in urology; Pharmaceutical preparations for wounds; Pharmaceutical preparations, namely, a blood clotting aid and delivery system for use in human and veterinary medicine; Pharmaceutical preparations, namely, a drug delivery system comprising polymer-based oral tablets for the continuous release of a wide variety of therapeutic agents; Pharmaceutical preparations, namely, a topical preparation for the treatment of ocular disorders prescribed by ophthalmologists, eye surgeons, and optometrists; Pharmaceutical preparations, namely, an analgesic for human consumption taken orally; Pharmaceutical preparations, namely, anticoagulants; Pharmaceutical preparations, namely, antidepressants; Pharmaceutical preparations, namely, appetite suppressants; Pharmaceutical preparations, namely, dantrolene sodium for injection; Pharmaceutical preparations, namely, injectable contraceptives; Pollen dietary supplements; Powdered nutritional supplement concentrate; Powdered nutritional supplement drink mix; Powdered nutritional supplement drink mix and concentrate; Propolis dietary supplements; Protein dietary supplements; Protein supplement shakes; Protein supplement shakes for weight gain purposes; Protein supplements; Protein supplements for animals; Royal jelly dietary supplements; Soy protein for use as a nutritional supplement in various powdered and ready-to-drink beverages; Vegan liquid protein supplements; Vegan protein for use as a nutritional supplement in ready-to-drink beverages; Veterinary preparations, namely, antioxidants; Vitamin and mineral supplements; Vitamin supplement in tablet form for use in making an effervescent beverage when added to water; Vitamin supplements; Vitamins and dietary food supplements for animals; Weight management supplements; Wheat for use as a dietary supplement; Wheat germ dietary supplements; Whey protein supplements; Yeast dietary supplements; Zinc supplement lozenges ]
Classification Information
International Class
005 - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides. - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides.
US Class Codes
006, 018, 044, 046, 051, 052
Class Status Code
6 - Active
Class Status Date
2013-09-16
Primary Code
005
First Use Anywhere Date
2008-05-08
First Use In Commerce Date
2008-08-06
Current Trademark Owners
Party Name
Party Type
30 - Original Registrant
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Trademark Owner History
Party Name
Party Type
30 - Original Registrant
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
20 - Owner at Publication
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
10 - Original Applicant
Legal Entity Type
03 - Corporation
Address
Please log in with your Justia account to see this address.
Correspondences
Name
L. S. VANLANDINGHAM III
Address
Please log in with your Justia account to see this address.
Trademark Events
| Event Date | Event Description |
| 2013-09-11 | NEW APPLICATION ENTERED IN TRAM |
| 2013-09-16 | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM |
| 2013-12-18 | ASSIGNED TO EXAMINER |
| 2013-12-28 | NON-FINAL ACTION WRITTEN |
| 2013-12-28 | NON-FINAL ACTION E-MAILED |
| 2013-12-28 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2014-01-08 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2014-01-21 | ASSIGNED TO LIE |
| 2014-01-23 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2014-01-23 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2014-02-05 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| 2014-02-05 | LAW OFFICE PUBLICATION REVIEW COMPLETED |
| 2014-02-19 | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED |
| 2014-03-11 | PUBLISHED FOR OPPOSITION |
| 2014-03-11 | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED |
| 2014-05-27 | REGISTERED-PRINCIPAL REGISTER |
| 2019-05-27 | COURTESY REMINDER - SEC. 8 (6-YR) E-MAILED |
| 2020-05-26 | TEAS SECTION 8 RECEIVED |
| 2020-09-09 | CASE ASSIGNED TO POST REGISTRATION PARALEGAL |
| 2020-09-09 | REGISTERED - SEC. 8 (6-YR) ACCEPTED |
| 2020-09-09 | NOTICE OF ACCEPTANCE OF SEC. 8 - E-MAILED |