ALLEGRO ICLINICAL - Trademark Details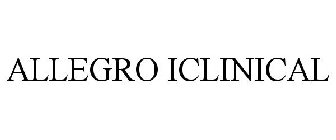
Status: 606 - Abandoned - No Statement Of Use Filed
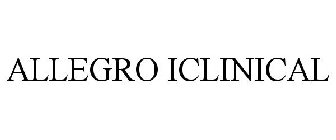
Serial Number
85706106
Word Mark
ALLEGRO ICLINICAL
Status
606 - Abandoned - No Statement Of Use Filed
Status Date
2014-05-12
Filing Date
2012-08-17
Mark Drawing
4000 - Standard character mark
Typeset
Published for Opposition Date
2013-08-13
Attorney Name
Law Office Assigned Location Code
M50
Employee Name
KHAN, ASMAT A
Statements
Indication of Colors claimed
Color is not claimed as a feature of the mark.
Disclaimer with Predetermined Text
"ICLINICAL"
Goods and Services
Managing databases for use by pharmaceutical companies and others in the design and administration of scientific and medical research projects, and of clinical trials for pharmaceuticals, medical devices and medical procedures; Managing databases for use by pharmaceutical companies and others in the data collection, data sharing, information exchange, and collaboration regarding scientific and medical research projects, and regarding clinical trials for pharmaceuticals, medical devices and medical procedures
Goods and Services
Providing on-line non-downloadable software for use in design and administration of scientific and medical research projects, and of clinical trials for pharmaceuticals, medical devices and medical procedures; providing a website featuring non-downloadable software for use in the design and administration of scientific and medical research projects, and of clinical trials for pharmaceuticals, medical devices and medical procedures; Hosting, managing, developing, and maintaining web sites, software, and applications for use by pharmaceutical companies and others in the design and administration of scientific and medical research projects, and of clinical trials for pharmaceuticals, medical devices and medical procedures; Providing on-line non-downloadable software for use in data collection, data sharing, information exchange, and collaboration regarding scientific and medical research projects, and regarding clinical trials for pharmaceuticals, medical devices and medical procedures, providing a website featuring non-downloadable software for use in data collection, data sharing, information exchange, and collaboration regarding scientific and medical research projects, and regarding clinical trials for pharmaceuticals, medical devices and medical procedures; Hosting, managing, developing, and maintaining web sites, software, applications, and databases for use by pharmaceutical companies and others in data collection, data sharing, information exchange, and collaboration regarding scientific and medical research projects, and regarding clinical trials for pharmaceuticals, medical devices and medical procedures; Providing on-line non-downloadable software for use by pharmaceutical companies and others in the tracking and management of patients, specimens, equipment, materials, funding, schedules, and performance milestones and metrics involved in scientific and medical research, and in clinical trials for pharmaceuticals, medical devices and medical procedures, providing a website featuring non-downloadable software for use by pharmaceutical companies and others in the tracking and management of patients, specimens, equipment, materials, funding, schedules, and performance milestones and metrics involved in scientific and medical research, and in clinical trials for pharmaceuticals, medical devices and medical procedures; Hosting, managing, developing, and maintaining web sites, software, and applications for use by pharmaceutical companies and others in the tracking and management of patients, specimens, equipment, materials, funding, schedules, and performance milestones and metrics involved in scientific and medical research, and in clinical trials for pharmaceuticals, medical devices and medical procedures; Providing on-line non-downloadable software for use by pharmaceutical companies and others in the monitoring and reporting of regulatory, standards, and safety compliance in scientific and medical research, and in clinical trials for pharmaceuticals, medical devices and medical procedures, providing a website featuring non-downloadable software for use by pharmaceutical companies and others in the monitoring and reporting of regulatory, standards, and safety compliance in scientific and medical research, and in clinical trials for pharmaceuticals, medical devices and medical procedures; Hosting, managing, developing, and maintaining web sites, software, and applications for use by pharmaceutical companies and others in the monitoring and reporting of regulatory, standards, and safety compliance in scientific and medical research, and in clinical trials for pharmaceuticals, medical devices and medical procedures; consulting services regarding all of the foregoing
Classification Information
International Class
035 - Advertising; business management; business administration; office functions. - Advertising; business management; business administration; office functions.
US Class Codes
100, 101, 102
Class Status Code
6 - Active
Class Status Date
2013-06-17
Primary Code
035
International Class
042 - Scientific and technological services and research and design relating thereto; industrial analysis and research services; design and development of computer hardware and software; legal services. - Scientific and technological services and research and design relating thereto; industrial analysis and research services; design and development of computer hardware and software; legal services.
US Class Codes
100, 101
Class Status Code
6 - Active
Class Status Date
2012-08-24
Primary Code
042
Correspondences
Name
CRAIG A. FIESCHKO
Address
Please log in with your Justia account to see this address.
Prior Registrations
| Relationship Type | Reel Number |
| Prior Registration | 3990967 |
Trademark Events
| Event Date | Event Description |
| 2012-08-21 | NEW APPLICATION ENTERED IN TRAM |
| 2012-08-24 | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM |
| 2012-12-12 | ASSIGNED TO EXAMINER |
| 2012-12-15 | NON-FINAL ACTION WRITTEN |
| 2012-12-15 | NON-FINAL ACTION E-MAILED |
| 2012-12-15 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2013-06-16 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2013-06-16 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2013-06-17 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2013-06-19 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| 2013-07-10 | ASSIGNED TO LIE |
| 2013-07-10 | LAW OFFICE PUBLICATION REVIEW COMPLETED |
| 2013-07-24 | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED |
| 2013-08-13 | PUBLISHED FOR OPPOSITION |
| 2013-08-13 | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED |
| 2013-10-08 | NOA E-MAILED - SOU REQUIRED FROM APPLICANT |
| 2014-05-12 | ABANDONMENT - NO USE STATEMENT FILED |
| 2014-05-12 | ABANDONMENT NOTICE MAILED - NO USE STATEMENT FILED |