MABIMMUNE - Trademark Details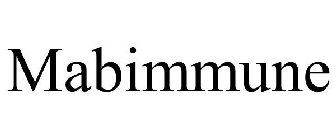
Status: 700 - Registered
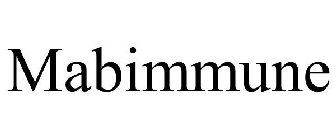
Serial Number
85683762
Registration Number
4896966
Word Mark
MABIMMUNE
Status
700 - Registered
Status Date
2016-02-09
Filing Date
2012-07-22
Registration Number
4896966
Registration Date
2016-02-09
Mark Drawing
4000 - Standard character mark
Typeset
Published for Opposition Date
2015-11-24
Attorney Name
Law Office Assigned Location Code
L20
Employee Name
VAGHANI, MAYUR C
Statements
Indication of Colors claimed
Color is not claimed as a feature of the mark.
Goods and Services
Chemicals, namely, chemical preparations containing monoclonal antibodies for biological assays; industrial chemicals containing monoclonal antibodies; chemical preparations containing monoclonal antibodies for scientific purposes; chemical preparations containing monoclonal antibodies for use in photography; biological materials, namely, biological tissue and cell cultures containing monoclonal antibodies other than for medical or veterinary purposes, inoculants in the nature of biological microorganisms containing monoclonal antibodies used for biomedical research; biological preparations, namely, biological tissue and cell cultures containing monoclonal antibodies other than for medical or veterinary purposes, inoculants containing monoclonal antibodies in the nature of biological microorganisms used for biomedical research; peptides for chemicals and foodstuff, namely, peptide substrates containing monoclonal antibodies used in analyzing and detecting certain biomarkers for laboratory or research use; polypeptides, namely, peptide substrates containing monoclonal antibodies used in analyzing and detecting certain biomarkers for laboratory or research use, protein antibodies, namely, monoclonal antibodies containing monoclonal antibodies for in vitro or in-vivo scientific or research use, antibody reagents containing monoclonal antibodies used for the detection of antigens in cell and tissue analysis for in vitro diagnostic use; diagnostic preparations containing monoclonal antibodies for scientific use; Biological compounds, namely, epitopes containing monoclonal antibodies in the nature of enzyme or mis-folded proteins for use in the pre-clinical research and development of pharmaceutical preparations such as antibodies or active peptides
Goods and Services
Skin care preparations containing monoclonal antibodies, namely, chemical peels for skin, anti-aging topical creams, anti-wrinkle topical creams, non-medicated lotions which are indicated specifically for reducing scar formation, topical ointments containing monoclonal antibodies indicated for accelerating the healing process or alleviating the symptoms of burns, namely, non-medicated ointments for the prevention and treatment of sunburn, topical beauty products, namely, cosmetics containing monoclonal antibodies
Goods and Services
Pharmaceutical preparations containing monoclonal antibodies for keloid scar formation, dermal burns, lymphomas, sarcomas, carcinomas, and leukemias, inflammatory disease, cardiovascular disease, atherosclerosis, rheumatoid arthritis, stroke or an acute coronary syndrome such as myocardial infarction heart attack, chronic liver disease, cerebral venous thrombosis, deep venous thrombosis or pulmonary embolism, arterial thrombosis, aneurysm, heart failure, ischemic heart diseases, angina pectoris, sudden cardiac death, hypertensive heart disease, non-coronary vessel disease, such as atherosclerosis, small vessel disease, vascular dementia, nephropathy, hypertriglyceridemia, hypercholesterolemia, hyperlipidemia, xanthomatosis, asthma, hypertension, emphysema, chronic pulmonary disease, cardiomyopathies and a cardiovascular condition associated with an interventional procedure such as restenosis following angioplasty, placement of a stent, synthetic or neutral extension grafts; pharmaceutical preparations containing monoclonal antibodies for the treatment of metabolic diseases including diabetes mellitus; veterinary preparations containing monoclonal antibodies for keloid scar formation, dermal burns, lymphomas, sarcomas, carcinomas, and leukemias, inflammatory disease, cardiovascular disease, atherosclerosis, rheumatoid arthritis, stroke or an acute coronary syndrome such as myocardial infarction heart attack, chronic liver disease, cerebral venous thrombosis, deep venous thrombosis or pulmonary embolism, arterial thrombosis, aneurysm, heart failure, ischemic heart diseases, angina pectoris, sudden cardiac death, hypertensive heart disease, non-coronary vessel disease, such as atherosclerosis, small vessel disease, vascular dementia, nephropathy, hypertriglyceridemia, hypercholesterolemia, hyperlipidemia, xanthomatosis, asthma, hypertension, emphysema, chronic pulmonary disease, cardiomyopathies and a cardiovascular condition associated with an interventional procedure such as restenosis following angioplasty, placement of a stent, synthetic or neutral extension grafts; veterinary preparations containing monoclonal antibodies for metabolic diseases including diabetes mellitus; tumor suppressing agents containing monoclonal antibodies; preparations containing monoclonal antibodies for treating neurodegenerative diseases; immunogenics, namely, pharmaceutical preparations containing monoclonal antibodies for inducing immune responses for the treatment of oncology indications including lymphomas, sarcomas, carcinomas, leukemias, bacterial infections, parasitic disease; vaccines containing monoclonal antibodies; antibodies containing monoclonal antibodies, namely, for treatment of anti-inflammatory, cardiovascular, oncological, and dermatological indications; active epitopes in the form of a pharmaceutical preparation containing monoclonal antibodies which modulate the biological activity of drug targets in-vivo; synthetic peptides containing monoclonal antibodies for pharmaceutical purposes for the treatment of keloid scar formation, dermal burns, lymphomas, sarcomas, carcinomas, and leukemias, inflammatory disease, cardiovascular disease, atherosclerosis, rheumatoid arthritis, stroke or an acute coronary syndrome such as myocardial infarction heart attack, chronic liver disease, cerebral venous thrombosis, deep venous thrombosis or pulmonary embolism, arterial thrombosis, aneurysm, heart failure, ischemic heart diseases, angina pectoris, sudden cardiac death, hypertensive heart disease, non-coronary vessel disease, such as atherosclerosis, small vessel disease, vascular dementia, nephropathy, hypertriglyceridemia, hypercholesterolemia, hyperlipidemia, xanthomatosis, asthma, hypertension, emphysema, chronic pulmonary disease, cardiomyopathies and a cardiovascular condition associated with an interventional procedure such as restenosis following angioplasty, placement of a stent, synthetic or neutral extension grafts; synthetic peptides for pharmaceutical purposes containing monoclonal antibodies for the treatment of metabolic diseases including diabetes mellitus; sanitary preparations containing monoclonal antibodies for medical purposes; biological preparations for medical purposes containing monoclonal antibodies for the treatment of keloid scar formation, dermal burns, lymphomas, sarcomas, carcinomas, and leukemias, inflammatory disease, cardiovascular disease, atherosclerosis, rheumatoid arthritis, stroke or an acute coronary syndrome such as myocardial infarction heart attack, chronic liver disease, cerebral venous thrombosis, deep venous thrombosis or pulmonary embolism, arterial thrombosis, aneurysm, heart failure, ischemic heart diseases, angina pectoris, sudden cardiac death, hypertensive heart disease, non-coronary vessel disease, such as atherosclerosis, small vessel disease, vascular dementia, nephropathy, hypertriglyceridemia, hypercholesterolemia, hyperlipidemia, xanthomatosis, asthma, hypertension, emphysema, chronic pulmonary disease, cardiomyopathies and a cardiovascular condition associated with an interventional procedure such as restenosis following angioplasty, placement of a stent, synthetic or neutral extension grafts; biological preparations for medical purposes containing monoclonal antibodies for the treatment of metabolic diseases including diabetes mellitus; biocides containing monoclonal antibodies; diagnostic preparations for medical or veterinary purposes containing monoclonal antibodies; sanitary towels containing monoclonal antibodies; dietetic food, namely, pasta, crackers, bread adapted for medical purposes containing monoclonal antibodies; dietetic beverages, namely, tea, vitamin fortified beverages, protein shakes adapted for medical purposes containing monoclonal antibodies; algaecide chemicals containing monoclonal antibodies for swimming pools; biological tissue containing monoclonal antibodies such as bone, skin, blood vessels, cartilage, muscle, teeth, extracellular matrix, and heart valve tissue intended for subsequent implantation; synthetic peptides and polypeptides containing monoclonal antibodies for the treatment of keloid scar formation, dermal burns, lymphomas, sarcomas, carcinomas, and leukemias, inflammatory disease, cardiovascular disease, atherosclerosis, rheumatoid arthritis, stroke or an acute coronary syndrome such as myocardial infarction heart attack, chronic liver disease, cerebral venous thrombosis, deep venous thrombosis or pulmonary embolism, arterial thrombosis, aneurysm, heart failure, ischemic heart diseases, angina pectoris, sudden cardiac death, hypertensive heart disease, non-coronary vessel disease, such as atherosclerosis, small vessel disease, vascular dementia, nephropathy, hypertriglyceridemia, hypercholesterolemia, hyperlipidemia, xanthomatosis, asthma, hypertension, emphysema, chronic pulmonary disease, cardiomyopathies and a cardiovascular condition associated with an interventional procedure such as restenosis following angioplasty, placement of a stent, synthetic or neutral extension grafts; synthetic peptides and polypeptides for the treatment of metabolic diseases including diabetes mellitus; chemicals, namely, chemical preparations for pharmaceutical or medical purposes containing monoclonal antibodies, namely, for keloid scar formation, dermal burns, lymphomas, sarcomas, carcinomas, and leukemias, inflammatory disease, cardiovascular disease, atherosclerosis, rheumatoid arthritis, stroke or an acute coronary syndrome such as myocardial infarction heart attack, chronic liver disease, cerebral venous thrombosis, deep venous thrombosis or pulmonary embolism, arterial thrombosis, aneurysm, heart failure, ischemic heart diseases, angina pectoris, sudden cardiac death, hypertensive heart disease, non-coronary vessel disease, such as atherosclerosis, small vessel disease, vascular dementia, nephropathy, hypertriglyceridemia, hypercholesterolemia, hyperlipidemia, xanthomatosis, asthma, hypertension, emphysema, chronic pulmonary disease, cardiomyopathies and a cardiovascular condition associated with an interventional procedure such as restenosis following angioplasty, placement of a stent, synthetic or neutral extension grafts; pharmaceutical preparations, namely, medicated scar-reducing lotions containing monoclonal antibodies; burn relief medication ointments containing monoclonal antibodies; Chemicals, namely, chemical preparations containing monoclonal antibodies for medical kits and tests, namely, diagnostic blood tests, companion diagnostics, and blood tests to determine the efficacy of the treatment strategy including both plasma and serum based assays for metabolic, cardiovascular, oncological, and inflammatory diseases
Goods and Services
Physical examination featuring monoclonal antibodies, namely, physical fitness training services, physical performance monitoring in the nature of physical fitness consultation
Goods and Services
Testing, inspection or research of pharmaceuticals, cosmetics or foodstuffs containing monoclonal antibodies; consultancy and providing information of testing, inspection or research of pharmaceuticals, cosmetics or foodstuffs containing monoclonal antibodies; testing, inspection or research of medical treatment and technique containing monoclonal antibodies; research in the field of biotechnology and antibodies containing monoclonal antibodies; providing information of testing, inspection or research of medical treatment and technique containing monoclonal antibodies; scientific research of health care related to monoclonal antibodies; testing, inspection or research of biology related to monoclonal antibodies; computer software design, computer programming, or maintenance of computer software featuring monoclonal antibodies; computer hardware design and development related to monoclonal antibodies; computer software design and development related to monoclonal antibodies; consultancy and providing information in the field of development of pharmaceutical preparations containing monoclonal antibodies; consultancy and providing information in the field of biomedical research related to monoclonal antibodies
Goods and Services
Medical services featuring monoclonal antibodies; medical services featuring monoclonal antibodies in the field of immunity, cardiology, rheumatology, oncology, pneumology, neurology; providing medical information featuring monoclonal antibodies; medical services, namely, conducting physical medical examinations featuring monoclonal antibodies in the fields of keloid scar formation, dermal burns, lymphomas, sarcomas, carcinomas, and leukemias, inflammatory disease, cardiovascular disease, atherosclerosis, rheumatoid arthritis, stroke or an acute coronary syndrome such as myocardial infarction heart attack, chronic liver disease, cerebral venous thrombosis, deep venous thrombosis or pulmonary embolism, arterial thrombosis, aneurysm, heart failure, ischemic heart diseases, angina pectoris, sudden cardiac death, hypertensive heart disease, non-coronary vessel disease, such as atherosclerosis, small vessel disease, vascular dementia, nephropathy, hypertriglyceridemia, hypercholesterolemia, hyperlipidemia, xanthomatosis, asthma, hypertension, emphysema, chronic pulmonary disease, cardiomyopathies and a cardiovascular condition associated with an interventional procedure such as restenosis following angioplasty, placement of a stent, synthetic or neutral extension grafts; medical services featuring monoclonal antibodies, namely, conducting physical medical examinations in the fields of metabolic diseases including diabetes mellitus; preparation and dispensing of medications containing monoclonal antibodies; dietary and nutritional guidance featuring monoclonal antibodies; providing information of health care featuring monoclonal antibodies; consulting in the field of medicine, occupational therapy, and nutrition featuring monoclonal antibodies; veterinary services featuring monoclonal antibodies; beauty salons featuring monoclonal antibodies; animal sanitary services, namely, animal grooming services, animal health care, and veterinary services in the nature of euthanasia of animals featuring monoclonal antibodies; health care featuring monoclonal antibodies; consultancy and providing information in the fields of medical imaging, diagnostic properties of pharmaceuticals, and medical care related to human physiology and pathophysiology featuring monoclonal antibodies; pharmaceutical consultation featuring monoclonal antibodies; providing information relating to diagnostic, prophylactic and therapeutic properties of pharmaceuticals featuring monoclonal antibodies; physical performance monitoring in the nature of physical therapy or physiotherapy services featuring monoclonal antibodies; ergometry, namely, nutrition counseling featuring monoclonal antibodies; ergometry in the nature of health assessment, namely, blood tests featuring monoclonal antibodies for the evaluation of physical fitness or response to training
Translation of Words in Mark
The wording "MABIMMUNE" has no meaning in a foreign language.
Classification Information
International Class
001 - Chemicals used in industry, science and photography, as well as in agriculture, horticulture and forestry; unprocessed artificial resins, unprocessed plastics; manures; fire extinguishing compositions; tempering and soldering preparations; chemical substances for preserving foodstuffs; tanning substances; adhesives used in industry. - Chemicals used in industry, science and photography, as well as in agriculture, horticulture and forestry; unprocessed artificial resins, unprocessed plastics; manures; fire extinguishing compositions; tempering and soldering preparations; chemical substances for preserving foodstuffs; tanning substances; adhesives used in industry.
US Class Codes
001, 005, 006, 010, 026, 046
Class Status Code
6 - Active
Class Status Date
2012-07-27
Primary Code
001
International Class
003 - Bleaching preparations and other substances for laundry use; cleaning, polishing, scouring and abrasive preparations; soaps; perfumery, essential oils, cosmetics, hair lotions; dentifrices. - Bleaching preparations and other substances for laundry use; cleaning, polishing, scouring and abrasive preparations; soaps; perfumery, essential oils, cosmetics, hair lotions; dentifrices.
US Class Codes
001, 004, 006, 050, 051, 052
Class Status Code
6 - Active
Class Status Date
2013-05-16
Primary Code
003
International Class
005 - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides. - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides.
US Class Codes
006, 018, 044, 046, 051, 052
Class Status Code
6 - Active
Class Status Date
2012-07-27
Primary Code
005
International Class
041 - Education; providing of training; entertainment; sporting and cultural activities. - Education; providing of training; entertainment; sporting and cultural activities.
US Class Codes
100, 101, 107
Class Status Code
6 - Active
Class Status Date
2013-05-16
Primary Code
041
International Class
042 - Scientific and technological services and research and design relating thereto; industrial analysis and research services; design and development of computer hardware and software; legal services. - Scientific and technological services and research and design relating thereto; industrial analysis and research services; design and development of computer hardware and software; legal services.
US Class Codes
100, 101
Class Status Code
6 - Active
Class Status Date
2012-07-27
Primary Code
042
International Class
044 - Medical services; veterinary services; hygienic and beauty care for human beings or animals; agriculture, horticulture and forestry services. - Medical services; veterinary services; hygienic and beauty care for human beings or animals; agriculture, horticulture and forestry services.
US Class Codes
100, 101
Class Status Code
6 - Active
Class Status Date
2012-07-27
Primary Code
044
Current Trademark Owners
Party Name
Party Type
31 - 1st New Owner Entered After Registration
Legal Entity Type
25
Address
Please log in with your Justia account to see this address.
Trademark Owner History
Party Name
Party Type
31 - 1st New Owner Entered After Registration
Legal Entity Type
25
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
30 - Original Registrant
Legal Entity Type
99 - Other (aktiengesellschaft).
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
20 - Owner at Publication
Legal Entity Type
99 - Other (aktiengesellschaft).
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
10 - Original Applicant
Legal Entity Type
99 - Other (aktiengesellschaft).
Address
Please log in with your Justia account to see this address.
Correspondences
Name
Janice Housey
Address
Please log in with your Justia account to see this address.
Foreign Application Information
| Filing Date | Application Number | Country | Foreign Priority Claim In |
| Switzerland | False | ||
| Switzerland | False |
Trademark Events
| Event Date | Event Description |
| 2012-07-25 | NEW APPLICATION ENTERED IN TRAM |
| 2012-07-27 | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM |
| 2012-08-13 | TEAS VOLUNTARY AMENDMENT RECEIVED |
| 2012-08-13 | TEAS AMENDMENT ENTERED BEFORE ATTORNEY ASSIGNED |
| 2012-11-15 | ASSIGNED TO EXAMINER |
| 2012-11-16 | NON-FINAL ACTION WRITTEN |
| 2012-11-16 | NON-FINAL ACTION E-MAILED |
| 2012-11-16 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2013-05-15 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2013-05-15 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2013-05-16 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2013-06-06 | FINAL REFUSAL WRITTEN |
| 2013-06-06 | FINAL REFUSAL E-MAILED |
| 2013-06-06 | NOTIFICATION OF FINAL REFUSAL EMAILED |
| 2013-12-06 | EXPARTE APPEAL RECEIVED AT TTAB |
| 2013-12-06 | JURISDICTION RESTORED TO EXAMINING ATTORNEY |
| 2013-12-06 | EX PARTE APPEAL-INSTITUTED |
| 2013-12-06 | TEAS REQUEST FOR RECONSIDERATION RECEIVED |
| 2013-12-30 | ASSIGNED TO LIE |
| 2014-01-06 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2014-01-06 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2014-01-23 | EXAMINERS AMENDMENT -WRITTEN |
| 2014-01-23 | EXAMINERS AMENDMENT E-MAILED |
| 2014-01-23 | NOTIFICATION OF EXAMINERS AMENDMENT E-MAILED |
| 2014-01-23 | EXAMINER'S AMENDMENT ENTERED |
| 2014-01-23 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| 2014-01-25 | LAW OFFICE PUBLICATION REVIEW COMPLETED |
| 2014-02-10 | WITHDRAWN FROM PUB - OG REVIEW QUERY |
| 2014-02-27 | PREVIOUS ALLOWANCE COUNT WITHDRAWN |
| 2014-03-06 | NON-FINAL ACTION WRITTEN |
| 2014-03-06 | NON-FINAL ACTION E-MAILED |
| 2014-03-06 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2014-07-23 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2014-07-23 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2014-07-24 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2014-07-28 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| 2014-07-28 | EXPARTE APPEAL TERMINATED |
| 2014-07-31 | LAW OFFICE PUBLICATION REVIEW COMPLETED |
| 2014-08-13 | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED |
| 2014-09-02 | PUBLISHED FOR OPPOSITION |
| 2014-09-02 | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED |
| 2014-10-28 | NOA E-MAILED - SOU REQUIRED FROM APPLICANT |
| 2014-11-19 | TEAS DELETE 1(B) BASIS RECEIVED |
| 2015-01-15 | CASE ASSIGNED TO INTENT TO USE PARALEGAL |
| 2015-04-21 | TEAS EXTENSION RECEIVED |
| 2015-04-21 | EXTENSION 1 FILED |
| 2015-04-21 | EXTENSION 1 GRANTED |
| 2015-04-23 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| 2015-08-27 | REVIEW OF CORRESPONDENCE COMPLETE |
| 2015-10-09 | TEAS PETITION TO AMEND BASIS RECEIVED |
| 2015-10-14 | ASSIGNED TO PETITION STAFF |
| 2015-10-15 | PETITION TO DIRECTOR - CHANGE BASIS - GRANTED |
| 2015-10-16 | NOTICE OF ALLOWANCE CANCELLED |
| 2015-10-16 | NOA CANCELLED; REPUBLICATION REQUIRED |
| 2015-11-04 | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED |
| 2015-11-24 | PUBLISHED FOR OPPOSITION |
| 2015-11-24 | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED |
| 2016-02-09 | REGISTERED-PRINCIPAL REGISTER |
| 2016-03-10 | TEAS CHANGE OF CORRESPONDENCE RECEIVED |
| 2020-05-13 | AUTOMATIC UPDATE OF ASSIGNMENT OF OWNERSHIP |
| 2021-02-09 | COURTESY REMINDER - SEC. 8 (6-YR) E-MAILED |
| 2021-07-21 | TEAS REVOKE/APP/CHANGE ADDR OF ATTY/DOM REP RECEIVED |
| 2021-07-21 | ATTORNEY/DOM.REP.REVOKED AND/OR APPOINTED |
| 2021-07-21 | TEAS CHANGE OF DOMESTIC REPRESENTATIVES ADDRESS |
| 2021-07-21 | TEAS CHANGE OF CORRESPONDENCE RECEIVED |