MICROBAC - Trademark Details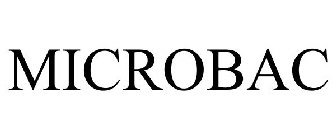
Status: 700 - Registered
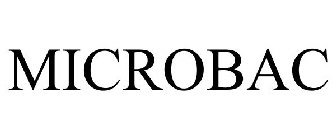
Serial Number
86698323
Registration Number
4986679
Word Mark
MICROBAC
Status
700 - Registered
Status Date
2016-06-28
Filing Date
2015-07-20
Registration Number
4986679
Registration Date
2016-06-28
Mark Drawing
4000 - Standard character mark
Typeset
Published for Opposition Date
2016-04-12
Attorney Name
Law Office Assigned Location Code
M30
Employee Name
DICKEY, ERICA G.J.
Statements
Certificate of Correction for Registration
In the statement, line 19, "containing microorganisms and cultures of microorganisms" should be should be inserted after "products".
Goods and Services
Cultures of micro-organisms, namely, lactic acid bacteria and bifidobacteria used in the manufacture of food and pharmaceutical products, dietetic foods, and dairy products; Active components of microbial strains for the manufacture of food and pharmaceutical products; Active components resulting from the processing of microorganisms, namely, metabolites obtained from the processing of probiotic bacterial cultures for the manufacture of food and pharmaceutical products; Micro-organisms, namely, bacteria for use in food and pharmaceutical manufacturing industry; Components of micro-organisms, namely, proteins, metabolites and enzyme for industrial manufacture of food and pharmaceutical products; Micro-organism cultures, namely, lactic acid bacteria and bifidobacteria having probiotic action on the organisms of humans for use in food and pharmaceutical industry
Goods and Services
Micro-organisms culture for medical use, namely, lactic acid bacteria and bifidobacteria having probiotic activity on humans for the treatment of diseases, namely, intestinal disorders, diarrhea and to re-equilibrate the intestinal bacterial flora, for the treatment of intestinal inflammations, inflammatory bowel diseases and inflammations of the lower gastrointestinal tract, for the treatment of ulcerative colitis and Crohn's disease in which inflammation is present both in the small intestine and in the colon; Pharmaceutical products containing micro-organisms cultures, namely, lactic acid bacteria and bifidobacteria having probiotic activity on humans for the treatment of diseases, namely, intestinal disorders, diarrhea and to re-equilibrate the intestinal bacterial flora, for the treatment of intestinal inflammations, inflammatory bowel diseases and inflammations of the lower gastrointestinal tract, for the treatment of ulcerative colitis and Crohn's disease in which inflammation is present both in the small intestine and in the colon; Pharmaceutical products containing microorganisms and cultures of microorganisms, namely, probiotic lactic acid bacteria, probiotic bifidobacteria which are able to ferment and use the fibres in the human organism for the treatment of diseases, namely, intestinal disorders, diarrhea and to re-equilibrate the intestinal bacterial flora, for the treatment of intestinal inflammations, inflammatory bowel diseases and inflammations of the lower gastrointestinal tract, for the treatment of ulcerative colitis and Crohn's disease in which inflammation is present both in the small intestine and in the colon, and dietary and nutritional supplements containing microorganisms and cultures of microorganisms, namely, probiotic lactic acid bacteria, probiotic bifidobacteria which are able to ferment and use the fibres in the human organism; Pharmaceutical products for oral consumption, namely, capsules, tablets, solutions, suspensions, granules, and pellets in dried form, containing micro-organisms cultures, namely, lactic acid bacteria and bifidobacteria having probiotic activity on humans for the treatment of diseases, including, namely, intestinal disorders, diarrhea and to re-equilibrate the intestinal bacterial flora, for the treatment of intestinal inflammations, inflammatory bowel diseases and inflammations of the lower gastrointestinal tract, for the treatment of ulcerative colitis and Crohn's disease in which inflammation is present both in the small intestine and in the colon, and dietary and nutritional supplements for oral consumption, namely, capsules, tablets, solutions, suspensions, granules, and pellets in dried form, containing micro-organisms cultures, namely, lactic acid bacteria and bifidobacteria having probiotic activity on humans; Microbial and biological preparations for improving the physical well-being of humans, namely, reducing inflammation and pain; Supplements, namely, dietary supplements, for medical use and baby food, all the above containing preparations and products resulting from the processing of microorganisms, particularly metabolites obtained from the processing of probiotic bacterial cultures; Supplements, namely, dietary supplements for medical use and baby food, all the above containing liquid, solid, dried or freeze-dried microorganisms or concentrated cultures of probiotic microorganisms; ferments for medical purposes, all the foregoing being for medical and veterinary use only
Pseudo Mark
MICRO BACK
Classification Information
International Class
005 - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides. - Pharmaceutical, veterinary and sanitary preparations; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides.
US Class Codes
006, 018, 044, 046, 051, 052
Class Status Code
6 - Active
Class Status Date
2015-07-24
Primary Code
005
Current Trademark Owners
Party Name
Party Type
30 - Original Registrant
Legal Entity Type
09 - Joint Stock Company
Address
Please log in with your Justia account to see this address.
Trademark Owner History
Party Name
Party Type
30 - Original Registrant
Legal Entity Type
09 - Joint Stock Company
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
20 - Owner at Publication
Legal Entity Type
09 - Joint Stock Company
Address
Please log in with your Justia account to see this address.
Party Name
Party Type
10 - Original Applicant
Legal Entity Type
09 - Joint Stock Company
Address
Please log in with your Justia account to see this address.
Correspondences
Name
JOHN P. MURTAUGH
Address
Please log in with your Justia account to see this address.
Foreign Application Information
| Filing Date | Application Number | Country | Foreign Priority Claim In |
| 2015-04-20 | 013964705 | EM | False |
| 2015-04-20 | 013964705 | Italy | True |
Trademark Events
| Event Date | Event Description |
| 2015-07-23 | NEW APPLICATION ENTERED IN TRAM |
| 2015-07-24 | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED IN TRAM |
| 2015-07-25 | NOTICE OF PSEUDO MARK E-MAILED |
| 2015-10-29 | ASSIGNED TO EXAMINER |
| 2015-10-30 | NON-FINAL ACTION WRITTEN |
| 2015-10-30 | NON-FINAL ACTION E-MAILED |
| 2015-10-30 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| 2016-01-13 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| 2016-01-26 | ASSIGNED TO LIE |
| 2016-01-28 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| 2016-01-28 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| 2016-03-02 | EXAMINERS AMENDMENT -WRITTEN |
| 2016-03-02 | EXAMINERS AMENDMENT E-MAILED |
| 2016-03-02 | NOTIFICATION OF EXAMINERS AMENDMENT E-MAILED |
| 2016-03-02 | EXAMINER'S AMENDMENT ENTERED |
| 2016-03-02 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| 2016-03-02 | ASSIGNED TO LIE |
| 2016-03-09 | LAW OFFICE PUBLICATION REVIEW COMPLETED |
| 2016-03-23 | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED |
| 2016-04-12 | PUBLISHED FOR OPPOSITION |
| 2016-04-12 | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED |
| 2016-06-28 | REGISTERED-PRINCIPAL REGISTER |
| 2016-08-01 | PAPER RECEIVED |
| 2016-10-31 | CASE ASSIGNED TO POST REGISTRATION PARALEGAL |
| 2016-08-01 | SEC 7 REQUEST FILED |
| 2016-10-31 | CORRECTION UNDER SECTION 7 ¿ PROCESSED |
| 2021-06-28 | COURTESY REMINDER - SEC. 8 (6-YR) E-MAILED |